What is CHCl3? Understanding Its Properties and Uses
What is CHCl3? Understanding Its Properties and Uses
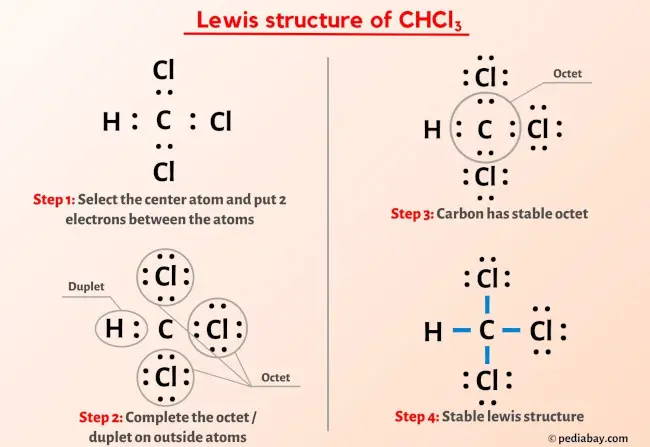
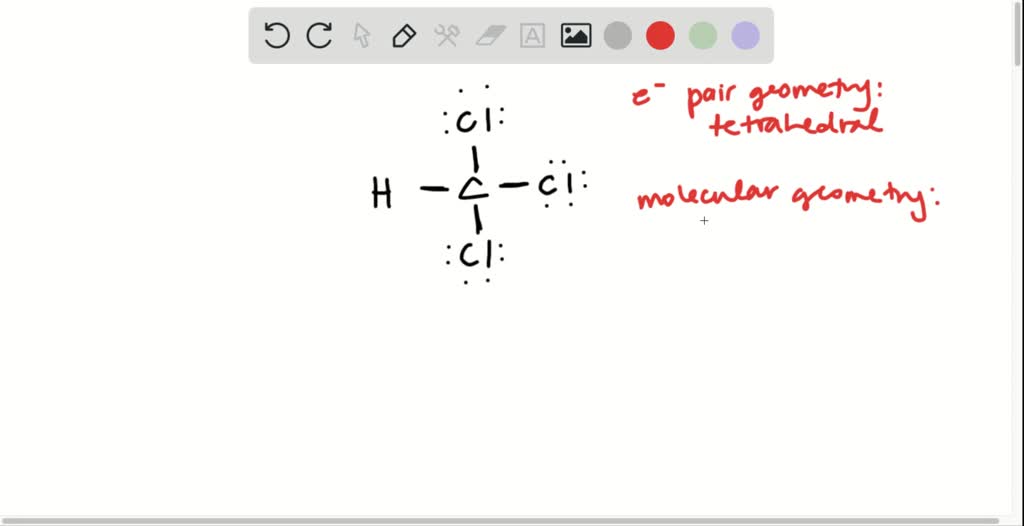
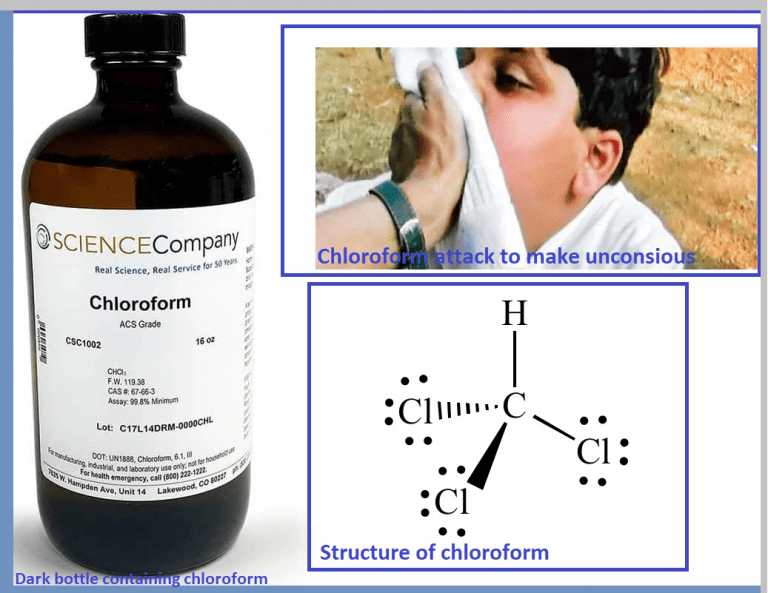
CHCl3, commonly known as chloroform, is a colorless, dense liquid with a sweet odor. It is a trihalomethane, composed of one carbon atom, one hydrogen atom, and three chlorine atoms. Widely recognized in chemistry, CHCl3 has diverse applications ranging from industrial processes to laboratory research. This post explores its properties, uses, and safety considerations to provide a comprehensive understanding of this compound.
Chemical Properties of CHCl3

CHCl3 is a polar solvent due to the electronegativity of chlorine atoms, making it effective for dissolving a wide range of organic compounds. Its molecular formula is CCl3H, and it has a molar mass of approximately 119.38 g/mol. Key properties include:
- Boiling Point: 61.2°C (142.2°F)
- Density: 1.489 g/cm³ (at 20°C)
- Solubility: Poorly soluble in water but miscible with organic solvents like ethanol and acetone.
📌 Note: CHCl3 is a volatile liquid, so proper ventilation is essential when handling it.
Common Uses of CHCl3

CHCl3’s versatility makes it valuable in various fields:
- Laboratory Solvent: Used in organic synthesis and extraction processes.
- Industrial Applications: Employed in the production of refrigerants and fluorocarbons.
- Historical Medical Use: Formerly used as an anesthetic but discontinued due to toxicity concerns.
| Application | Details |
|---|---|
| Solvent | Dissolves fats, oils, and many organic compounds. |
| Industrial Chemical | Used in manufacturing refrigerants and dyes. |
| Analytical Chemistry | Employed in chromatography and spectroscopy. |

Safety and Handling of CHCl3
While CHCl3 is useful, it poses health risks:
- Toxicity: Prolonged exposure can cause liver and kidney damage.
- Carcinogenicity: Classified as a potential carcinogen by the EPA.
- Storage: Store in a cool, well-ventilated area away from heat sources.
⚠️ Note: Always wear protective gear, including gloves and goggles, when working with CHCl3.
Environmental Impact of CHCl3

CHCl3 can contaminate water sources and soil, posing risks to ecosystems. Its production and disposal must adhere to strict regulations to minimize environmental harm.
Key Takeaways
- CHCl3 is a polar solvent with diverse applications in chemistry and industry.
- Its properties include a low boiling point and high density.
- Safety precautions are crucial due to its toxicity and potential health risks.
Checklist for Handling CHCl3 Safely
- Use in a well-ventilated area.
- Wear protective equipment (gloves, goggles, lab coat).
- Store away from heat and open flames.
- Dispose of according to local regulations.
What is the chemical formula of chloroform?
+The chemical formula of chloroform is CHCl3.
Is CHCl3 safe to use?
+CHCl3 is toxic and potentially carcinogenic. Proper safety measures are essential when handling it.
What are the main uses of CHCl3?
+CHCl3 is used as a solvent in laboratories, in industrial processes, and historically as an anesthetic.
CHCl3 remains a significant compound in chemistry and industry, but its handling requires caution. By understanding its properties and uses, you can ensure safe and effective application. (chloroform properties, chloroform uses, chemical safety, laboratory solvents)