Understanding the Sulfate Ion Charge: A Quick Guide
Understanding the sulfate ion charge is essential for anyone studying chemistry, environmental science, or even industries like water treatment and agriculture. The sulfate ion, represented as SO₄²⁻, plays a crucial role in various chemical processes and natural systems. This quick guide will break down its charge, properties, and significance in a way that’s easy to grasp, whether you’re a student, researcher, or professional. By the end, you’ll have a clear understanding of why the sulfate ion charge matters and how it impacts different fields. (sulfate ion charge, chemical properties, water treatment)
What is the Sulfate Ion?

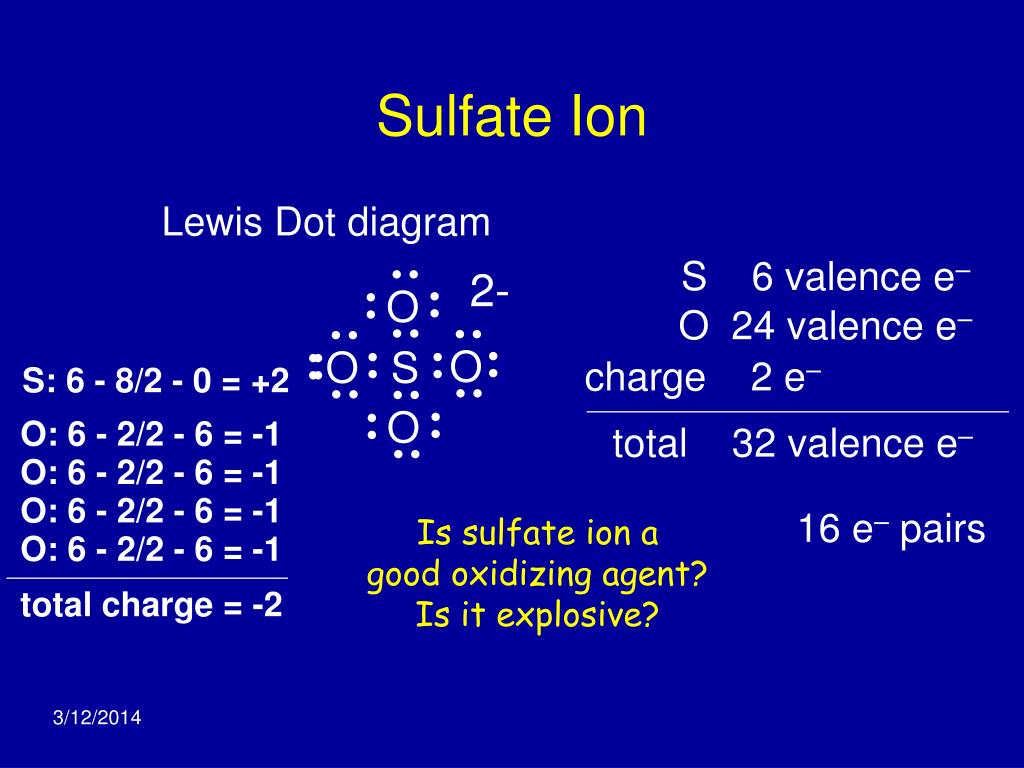
The sulfate ion (SO₄²⁻) is a polyatomic ion composed of one sulfur atom and four oxygen atoms. It carries a negative charge of -2, making it a key player in many chemical reactions. Sulfate ions are commonly found in minerals, soils, and natural water sources. They are also a byproduct of industrial processes, which highlights their importance in environmental monitoring. (polyatomic ion, sulfur, oxygen)
Why Does the Sulfate Ion Have a -2 Charge?

The -2 charge of the sulfate ion arises from its electron configuration. In the sulfate ion, sulfur forms double bonds with two oxygen atoms and single bonds with the other two, resulting in an overall negative charge. This charge is distributed across the ion, making it highly stable and reactive in chemical solutions. Understanding this charge is vital for predicting how sulfate ions interact with other substances, such as metals and cations. (electron configuration, chemical bonds, reactivity)
📌 Note: The sulfate ion’s charge makes it an excellent ligand in coordination chemistry, often forming complexes with metal ions.
Applications of the Sulfate Ion Charge

The sulfate ion charge has practical applications across various industries:
- Water Treatment: Sulfate ions are monitored to ensure water quality, as high levels can indicate contamination.
- Agriculture: Sulfates are essential nutrients for plants, often added as fertilizers.
- Chemical Manufacturing: Sulfate compounds are used in producing detergents, textiles, and pharmaceuticals.
These applications underscore the importance of understanding the sulfate ion charge in both scientific and industrial contexts. (water quality, fertilizers, chemical manufacturing)
How to Identify Sulfate Ions

Identifying sulfate ions in a solution is straightforward with the right tests. One common method is the barium chloride test, where adding barium chloride to a solution containing sulfate ions produces a white precipitate of barium sulfate. This simple test is widely used in laboratories and field studies. (barium chloride test, laboratory techniques)
| Test | Reagent | Observation |
|---|---|---|
| Barium Chloride Test | BaCl₂ | White precipitate (BaSO₄) |

Key Takeaways: Sulfate Ion Charge Explained

To summarize, the sulfate ion charge is a fundamental concept with wide-ranging implications. Here’s a quick checklist to reinforce your understanding:
- ✅ The sulfate ion (SO₄²⁻) carries a -2 charge.
- ✅ It is composed of one sulfur atom and four oxygen atoms.
- ✅ Sulfate ions are crucial in water treatment, agriculture, and chemical manufacturing.
- ✅ The barium chloride test is a simple way to identify sulfate ions.
By mastering these points, you’ll be well-equipped to tackle problems related to the sulfate ion charge. (sulfate ion charge, chemical testing, industrial applications)
What is the charge of the sulfate ion?
+The sulfate ion (SO₄²⁻) has a -2 charge.
Why is the sulfate ion charge important in water treatment?
+The sulfate ion charge helps monitor water quality, as high sulfate levels can indicate contamination or industrial runoff.
How can you identify sulfate ions in a solution?
+The barium chloride test is commonly used to identify sulfate ions by producing a white precipitate of barium sulfate.
In closing, the sulfate ion charge is a foundational concept in chemistry with practical applications across multiple fields. Whether you’re analyzing water samples, studying plant nutrition, or working in chemical manufacturing, understanding this charge is invaluable. With this guide, you’re now better equipped to apply this knowledge in real-world scenarios. Keep exploring, and don’t hesitate to dive deeper into related topics like ion behavior or environmental chemistry to expand your expertise. (ion behavior, environmental chemistry, practical applications)



