Open vs. Closed Systems: Physics Explained Simply

In the world of physics, understanding the difference between open systems and closed systems is fundamental. These concepts not only shape our understanding of energy and matter but also have practical applications in engineering, environmental science, and everyday life. Whether you're a student, a professional, or simply curious, this guide breaks down the complexities into simple, digestible insights. Let’s explore how these systems work, their key differences, and why they matter, open systems vs. closed systems in physics, thermodynamics explained simply.
What Are Open and Closed Systems in Physics?

In physics, systems are categorized based on their interaction with their surroundings. An open system exchanges both matter and energy with its environment, while a closed system exchanges only energy, keeping matter constant. These definitions are rooted in thermodynamics, the branch of physics that studies energy and its transformations, energy exchange in systems, matter transfer in physics.
Key Differences Between Open and Closed Systems
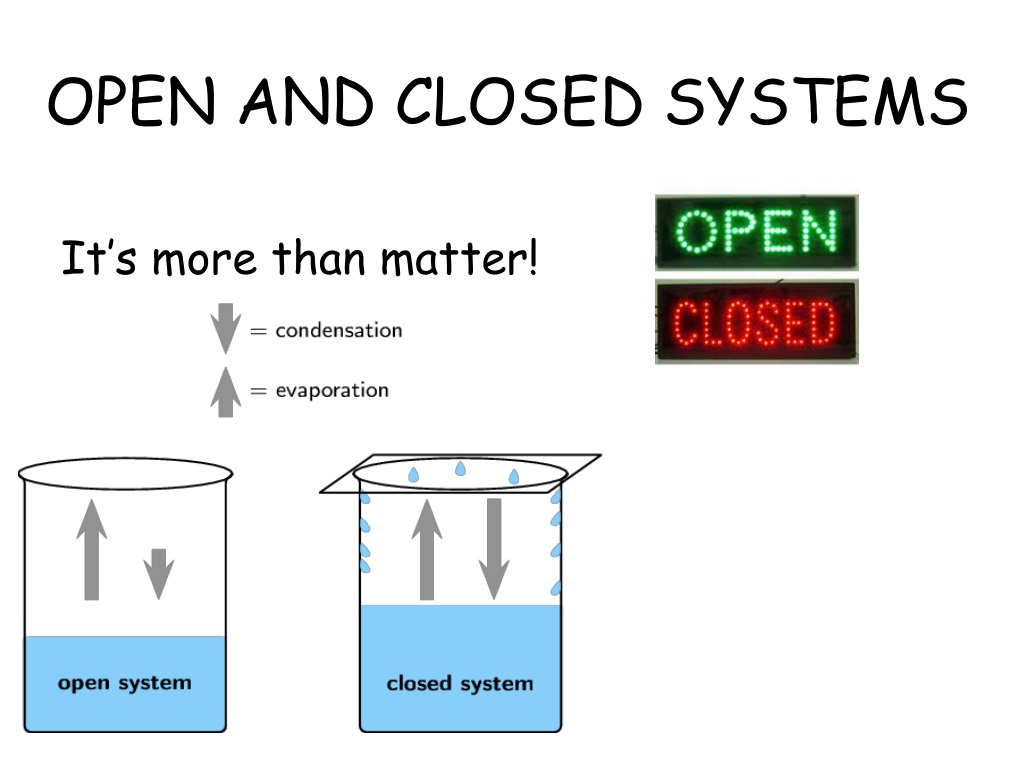
Open Systems: Characteristics and Examples
Open systems are dynamic and constantly interact with their surroundings. Examples include:
- Boiling water in an open pot (loses water vapor)
- Earth’s atmosphere (exchanges gases and heat)
- A car engine (releases exhaust gases and heat)
These systems are essential in understanding processes like heat transfer and mass flow, examples of open systems, dynamic systems in physics.
Closed Systems: Characteristics and Examples
Closed systems are more isolated, allowing only energy transfer. Common examples include:
- A sealed water bottle (no matter exchange)
- A gas in a rigid container (expands or contracts with temperature)
- The universe itself (assumed to be a closed system)
These systems are crucial in studying energy conservation and internal processes, examples of closed systems, isolated systems in thermodynamics.
Why Do These Systems Matter?

Understanding open and closed systems has practical implications. For instance:
- Engineers use these principles to design efficient machines.
- Environmental scientists study open systems to model climate change.
- Everyday phenomena like cooking or heating rely on these concepts.
📌 Note: While isolated systems (no exchange of energy or matter) are also studied, they are less common in real-world scenarios, practical applications of thermodynamics, real-world physics examples.
| Aspect | Open System | Closed System |
|---|---|---|
| Matter Exchange | Yes | No |
| Energy Exchange | Yes | Yes |
| Example | Boiling water in an open pot | Sealed water bottle |

Checklist: Understanding Open vs. Closed Systems

- Identify whether matter is exchanged with the environment.
- Determine if energy transfer occurs.
- Use real-world examples to solidify understanding.
- Apply these concepts to solve practical problems.
In summary, open systems and closed systems are foundational concepts in physics that help us understand how energy and matter interact. By grasping these differences, you can better analyze physical processes and apply them in various fields. Whether you're studying thermodynamics or simply curious about the world around you, this knowledge is invaluable, physics concepts simplified, thermodynamics for beginners.
What is the main difference between open and closed systems?
+
Open systems exchange both matter and energy with their surroundings, while closed systems only exchange energy, keeping matter constant, open vs. closed systems.
Can you give an example of an open system?
+
A boiling pot of water is an open system because it loses water vapor (matter) and heat (energy) to the environment, examples of open systems.
Why are closed systems important in thermodynamics?
+
Closed systems help study energy conservation and internal processes without the complexity of matter exchange, making them ideal for theoretical analysis, thermodynamics principles.