Unlocking Nitrile Carboxylic Acid Secrets: Uses & Benefits

Nitrile carboxylic acid, a versatile compound in organic chemistry, has been gaining attention for its wide-ranging applications across industries. From pharmaceuticals to agriculture, its unique properties make it an invaluable asset. This blog explores the uses and benefits of nitrile carboxylic acid, shedding light on why it’s becoming a cornerstone in modern chemical processes. Whether you’re a researcher, manufacturer, or simply curious, understanding this compound can unlock new possibilities. (nitrile carboxylic acid uses, nitrile carboxylic acid benefits)
What is Nitrile Carboxylic Acid?
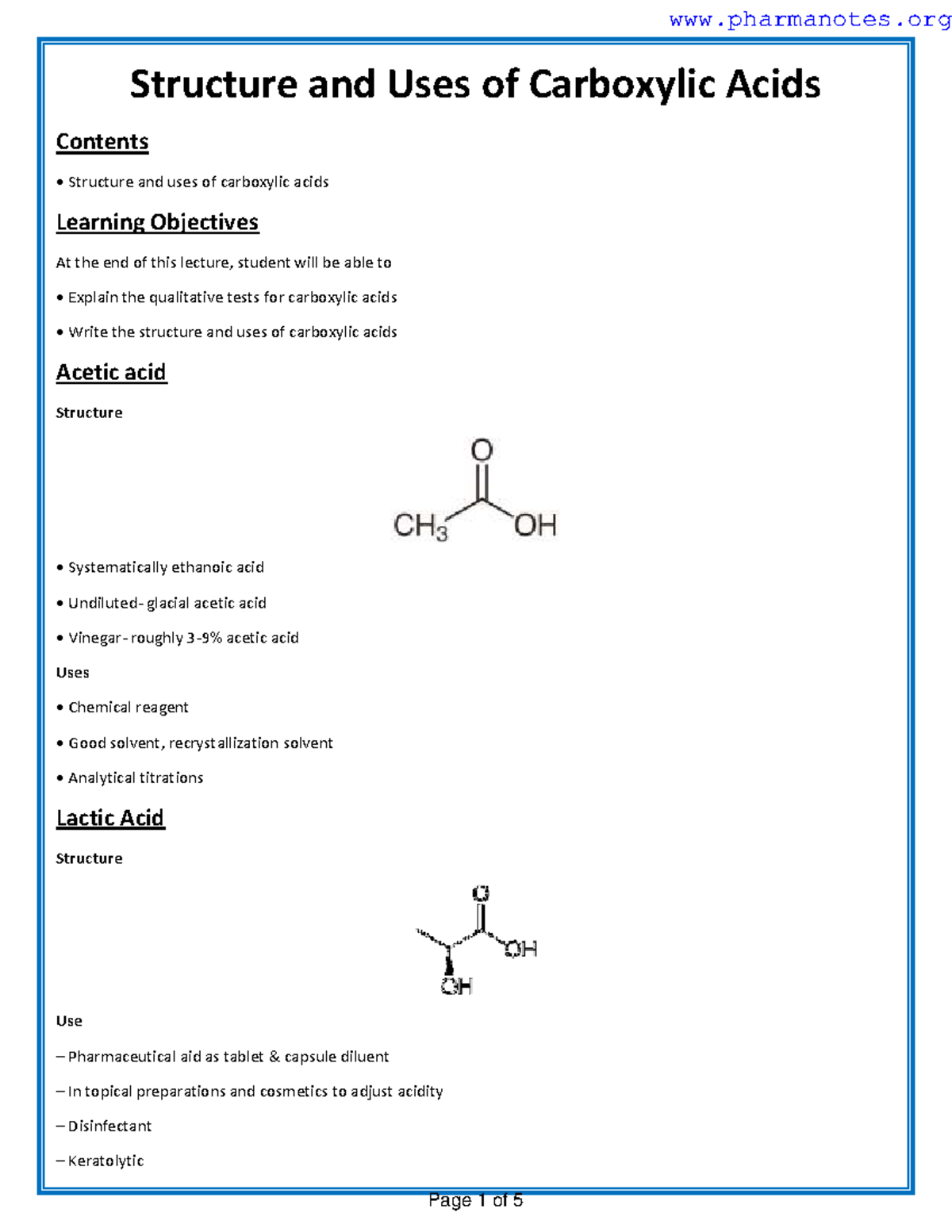
Nitrile carboxylic acid is an organic compound characterized by the presence of both a nitrile group (-CN) and a carboxylic acid group (-COOH). This dual functionality allows it to participate in various chemical reactions, making it highly versatile. Its structure enables it to act as a precursor in synthesizing pharmaceuticals, agrochemicals, and specialty chemicals. (nitrile carboxylic acid structure, nitrile carboxylic acid synthesis)
Key Uses of Nitrile Carboxylic Acid

Pharmaceutical Industry
In pharmaceuticals, nitrile carboxylic acid serves as a building block for creating active pharmaceutical ingredients (APIs). Its reactivity allows chemists to design complex molecules with therapeutic properties. Common applications include the production of anti-inflammatory drugs and antibiotics. (pharmaceutical applications, API synthesis)
Agriculture and Pesticides
The compound is also pivotal in developing agrochemicals, such as herbicides and fungicides. Its ability to bind to target molecules enhances the efficacy of pesticides, promoting crop protection and yield. (agrochemicals, pesticide formulation)
Specialty Chemicals
Nitrile carboxylic acid is used in manufacturing specialty chemicals, including dyes, adhesives, and coatings. Its stability and reactivity make it ideal for creating high-performance materials. (specialty chemicals, chemical synthesis)
Benefits of Nitrile Carboxylic Acid
Versatility in Reactions
The compound’s dual functional groups enable it to participate in a variety of reactions, making it a favorite among chemists. This versatility reduces the need for multiple reagents, streamlining processes. (chemical versatility, reaction efficiency)
Cost-Effectiveness
Nitrile carboxylic acid is relatively inexpensive to produce, making it an economical choice for large-scale manufacturing. Its efficiency in reactions further reduces overall production costs. (cost-effective chemicals, manufacturing efficiency)
Environmental Impact
Compared to other compounds, nitrile carboxylic acid often results in fewer byproducts, minimizing environmental impact. Its use aligns with sustainable chemistry practices. (sustainable chemistry, eco-friendly chemicals)
📌 Note: Always follow safety protocols when handling nitrile carboxylic acid, as it can be corrosive and harmful if not managed properly.
Summary and Checklist

Nitrile carboxylic acid is a powerhouse compound with diverse applications and benefits. To harness its potential, consider the following checklist:
- Identify the specific industry need (e.g., pharmaceuticals, agriculture)
- Assess the compound’s reactivity for your application
- Ensure compliance with safety and environmental regulations
- Optimize production processes for cost efficiency
Nitrile carboxylic acid’s unique properties and wide-ranging applications make it an indispensable tool in modern chemistry. By understanding its uses and benefits, industries can unlock new opportunities for innovation and growth. (nitrile carboxylic acid applications, chemical innovation)
What is nitrile carboxylic acid used for?
+
Nitrile carboxylic acid is used in pharmaceuticals, agrochemicals, and specialty chemicals due to its versatility in chemical reactions. (nitrile carboxylic acid uses)
Is nitrile carboxylic acid environmentally friendly?
+
Yes, it often produces fewer byproducts, making it a more sustainable option in chemical synthesis. (eco-friendly chemicals)
How is nitrile carboxylic acid synthesized?
+
It is typically synthesized through reactions involving nitriles and carboxylic acid derivatives. (nitrile carboxylic acid synthesis)