Acetylene Molecular Mass: Quick Facts and Calculation Guide
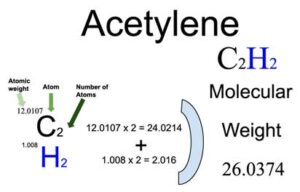
Acetylene, a colorless and highly flammable gas, is widely used in various industries, including welding, cutting, and chemical synthesis. Understanding its molecular mass is crucial for applications ranging from laboratory experiments to industrial processes. This blog post will guide you through the quick facts and calculation methods for acetylene’s molecular mass, ensuring you have the knowledge needed for both informational and practical purposes.
What is Acetylene and Its Molecular Formula?
Acetylene, also known as ethyne, has the molecular formula C₂H₂. It is the simplest alkyne hydrocarbon, consisting of two carbon atoms and two hydrogen atoms. Its unique structure makes it a valuable compound in organic chemistry and industrial applications.
Key Properties of Acetylene
- State: Gas at room temperature
- Flammability: Highly flammable
- Uses: Welding, cutting, chemical synthesis
How to Calculate Acetylene Molecular Mass

To calculate the molecular mass of acetylene, follow these steps:
Identify Atomic Masses:
- Carbon ©: 12.01 g/mol
- Hydrogen (H): 1.008 g/mol
- Carbon ©: 12.01 g/mol
Multiply by Atom Count:
- Carbon: 2 atoms × 12.01 g/mol = 24.02 g/mol
- Hydrogen: 2 atoms × 1.008 g/mol = 2.016 g/mol
- Carbon: 2 atoms × 12.01 g/mol = 24.02 g/mol
Sum the Values:
Total molecular mass = 24.02 g/mol + 2.016 g/mol = 26.036 g/mol
📌 Note: Always use the most accurate atomic masses for precise calculations.
Molecular Mass Table for Acetylene
| Element | Atomic Mass (g/mol) | Number of Atoms | Total Mass (g/mol) |
|---|---|---|---|
| Carbon (C) | 12.01 | 2 | 24.02 |
| Hydrogen (H) | 1.008 | 2 | 2.016 |
| Total Molecular Mass | 26.036 | ||

Why Knowing Acetylene’s Molecular Mass Matters

Understanding the molecular mass of acetylene is essential for:
- Stoichiometry calculations in chemical reactions.
- Safety precautions due to its high flammability.
- Industrial applications, such as determining gas volumes for welding.
Quick Checklist for Acetylene Molecular Mass Calculation
- Verify the molecular formula (C₂H₂).
- Use accurate atomic masses for carbon and hydrogen.
- Double-check your calculations to ensure precision.
Acetylene’s molecular mass of 26.036 g/mol is a fundamental piece of information for chemists, engineers, and students alike. By following the steps outlined above, you can confidently calculate and apply this value in various scenarios. Whether you’re conducting experiments or working in an industrial setting, this knowledge is invaluable.
What is the molecular formula of acetylene?
+The molecular formula of acetylene is C₂H₂.
How do you calculate the molecular mass of acetylene?
+Multiply the atomic masses of carbon (12.01 g/mol) and hydrogen (1.008 g/mol) by their respective atom counts, then sum the values to get 26.036 g/mol.
Why is acetylene’s molecular mass important?
+It’s crucial for stoichiometry, safety precautions, and industrial applications like welding and chemical synthesis.
acetylene molecular mass,acetylene molecular formula,acetylene uses,molecular mass calculation,acetylene properties,acetylene in welding,acetylene chemical synthesis,acetylene safety precautions